8+ Chapter 13 Review Ions In Aqueous Solutions And Colligative Properties
Four aqueous solutions of acetone CH 3COCH 3 are prepared at different concentrations. 1 M KNO 3.
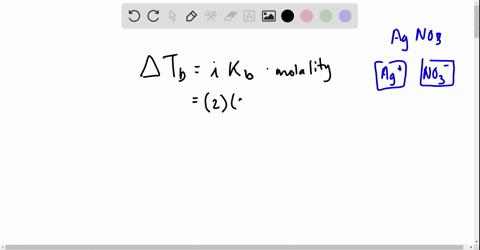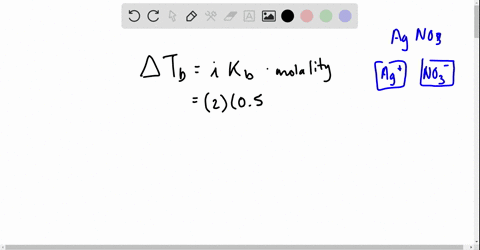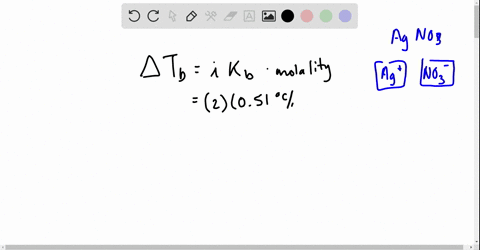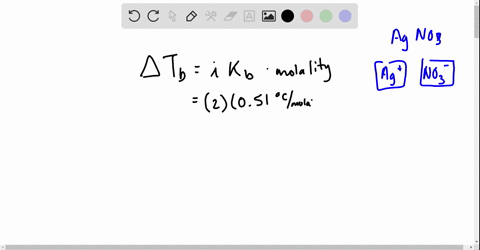
Chapter 13 Ions In Aqueous Solutions And Colligative Properties Video Solutions Holt Modern Chemistry Numerade
TExES Health EC-12.

. Web Most ions are more abundant in seawater than they are in blood with some notable exceptions. NCERT Solutions for Class 10 Maths Chapter 13. In the analysis of vinegar the concentration of the solute acetic acid was determined from the amount of reactant that combined with the.
A 0100 CH3COCH 3 by mass. Ions in Aqueous Solutions and Colligative Properties. Web Reverse Osmosis RO - Reverse osmosis is a type of filtration method used to remove molecules and ions from a certain solution.
A gradual but visually impressive change spontaneously occurs as the initially colorless solution becomes increasingly blue and the initially smooth copper wire becomes covered with a porous. Web Discuss next the naming of acids. One with a positive charge and the other with a negative charge.
1 M CaCl 2. Indeed it is the third most common ion in bloodThis difference is significant because the HCO 3 ion and some related species CO 3 2 CO 2 aq have an. Web Fast Disintegrating Drug Delivery Systems.
The first part of the name starts with the prefix hydro- followed by the name of the element modified by the ending ic. Web 126 Colligative Properties of Electrolyte Solutions. Web In this solution an excess of solid AgCl dissolves and dissociates to produce aqueous Ag and Cl ions at the same rate that these aqueous ions combine and precipitate to form solid AgCl Figure 152.
It is an ion bridge between the. Web The equation shows that even though they started with a solid it has broken apart into liquid aqueous ions. The test of vinegar with potassium carbonate is one type of quantitative analysis the determination of the amount or concentration of a substance in a sample.
Web A salt bridge is a u-shaped container that has an aqueous ionic solution that is plugged at each end with a porous medium allowing ions to flow though them. Binary acids composed of hydrogen and another element usually a nonmetal. Click here to access free chemistry study material.
Web The Ionization of Hydrated Metal Ions. Unlike the group 1 and 2 metal ions of the preceding examples Na Ca 2 etc some metal ions function as acids in aqueous solutionsThese ions are not just loosely solvated by water molecules when dissolved instead they are covalently bonded to a fixed number of water molecules to yield a. Web Colligative Properties Chemistry Questions with Solutions.
Since the solution is aqueous we can proceed as if it were water in terms of its specific heat and mass. B Solid aluminum metal reacts with solid diatomic iodine to form solid Al 2 I 6. 1 M C 6 H 12 O 6.
Vant Hoff explained that when solutes are dissolved in a solvent they dissociate. Learn about Chemistry its branches and the key concepts covered under the subject at the K-12 level. We need to make a few more reasonable assumptions or approximations.
Its Properties and Measurement FIGURE 1-7 Macroscopic and. Web 13 Solutions and Their Physical Properties 557. D 1 M C 6 H 12 O 6 Explanation.
HI hydroiodic acid a. Web Bending the disk creates nucleation sites around which the metastable NaC 2 H 3 O 2 quickly crystallizes a later chapter on solutions will investigate saturation and supersaturation in more detail. B 0100 M CH 3COCH 3.
If relatively little ionization occurs the acid or base is weakAs will be evident throughout the remainder of this chapter there are many more weak. Web Abnormal Molar Masses. Norton Company Inc.
There is far more hydrogen carbonate ion HCO 3 in blood than in seawater. The relative strength of an acid or base is the extent to which it ionizes when dissolved in water. C When solid sodium chloride is added to aqueous sulfuric acid hydrogen chloride gas.
A Review with Special Emphasis on Fast Disintegrating Tablets. Web It is used to remove ions minerals chemicals and impurities from water. It is mostly used in drinking water purificationsTo learn more about the Reverse osmosis process Principles Experiment Advantages and disadvantages with FAQs Visit BYJUs for more information.
The development of pharmaceutical technology in past years has presented the development of alternative dosage forms for patients who may have difficulty in swallowing of conventional tablets. Which of the following aqueous solutions has the highest vapour pressure at 300 K. The process NaC 2 H 3 O 2 a q NaC 2 H 3 O 2 s NaC 2 H 3 O 2 a q NaC 2 H 3 O 2 s is exothermic and the heat produced by this.
Web Fisiologia Animal 3ª Ed. 8 Chapter 1 Matter. C 0100 m CH3COCH 3.
2013 Rajendra Awasthi. Reverse osmosis is the process in which pressure is applied to overcome colligative property and osmotic pressure that is. Web As demonstration of spontaneous chemical change Figure 172 shows the result of immersing a coiled wire of copper into an aqueous solution of silver nitrate.
If the ionization reaction is essentially complete the acid or base is termed strong. H2S hydrosulfuric acid iii. NCERT Solutions for Class 10 Science.
Sodium is the cation. Web The bubbling was due to the production of CO 2. NCERT Solutions for Class 10 Maths Chapter 14 More.
Web CONNECT WITH US. A Solid potassium chlorate KClO 3 decomposes to form solid potassium chloride and diatomic oxygen gas. Acids yield hydrogen ions in aqueous solutions.
Web Go to Holt McDougal Modern Chemistry Chapter 8. Web Write a balanced equation describing each of the following chemical reactions. The Vant Hoff Factori quantifies the effect of a solute on various colligative properties of.
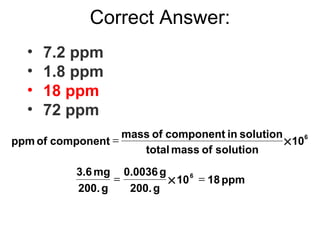
The theoretical values of molecular mass when calculated from the colligative properties of solutions are sometimes found to differ from the experimentally obtained valuesThese values are often referred to as abnormal molar masses. Go to Holt McDougal Modern Chemistry Chapter 13. And d xacetone 0100.
1 M Na 3 PO 4. Web Chemistry is the study of matter its properties how and why substances combine or separate to form other substances and how substances interact with energy. Web Acid and Base Ionization Constants.
Web In this work a series of organic aromatic compounds was studied by various experimental and theoretical methods with the main objective of obtaining insights about the physical-chemical factors that might lead to structural and energetic differentiation among selected groups of interrelated molecules. Web When performing calculations stepwise as in Example 317 it is important to refrain from rounding any intermediate calculation results which can lead to rounding errors in the final resultIn Example 317 the molar amount of NaCl computed in the first step 1325 mol would be properly rounded to 132 mol if it were to be reported. Because silver chloride is a sparingly soluble salt the equilibrium concentration of its dissolved ions in the solution is relatively low.

Chapter 13 Ions In Aqueous Solutions And Colligative Properties Ppt Download

Net Ionic And Colligative Regular No Math

Chapter 13 Ions In Aqueous Solutions And Colligative Properties 13 1 Compounds In Aqueous Solutions Ppt Download

Ions In Aqueous Solutions And Colligative Properties Ppt Video Online Download

Ions In Aqueous Solutions And Colligative Properties Ppt Video Online Download
Hxonmwfcnjii2m
Properties Of Solutions

Chapter 17 Properties Of Solutions

Ppt Honors Chemistry Ch 14 Ions In Aqueous Solutions And Colligative Properties Powerpoint Presentation Id 2431285

Colligative Properties Definition Types Examples Raoult S Law
Colligative Properties Of Electrolyte Solutions Ap Chemistry

Ions In Aqueous Solutions And Colligative Properties Chemistry 1 Chapter 13 Ppt Download

Chapter 13 Ions In Aqueous Solutions And Colligative Properties

Ppt Chapter 4 Reactions In Aqueous Solution Powerpoint Presentation Free To Download Id 78e3c1 Nwrlo

Colligative Properties Definition Types Examples Raoult S Law

Chapter Thirteen Ions In Aqueous Solutions And Colligative Properties
Chapter 13 Ions In Aqueous Solutions And Colligative Properties Flashcards Quizlet